A full portfolio of dosage strengths to suit your patient’s life1
3-times-a-week 40 mg/mL |
|
Once-daily 20 mg/mL |
Both Glatopa dosage strength options are FDA approved as therapeutically equivalent to Copaxone® (glatiramer acetate injection).1-3
- Once-daily Glatopa 20 mg/mL was approved as a generic substitute for Copaxone® in April 2015, and 3-times-a-week Glatopa 40 mg/mL was approved in early 20182,4
More injection-free days with 3-times-a-week dosing1*
Fewer injection days means more flexibility for your patients compared with once-daily formulations.
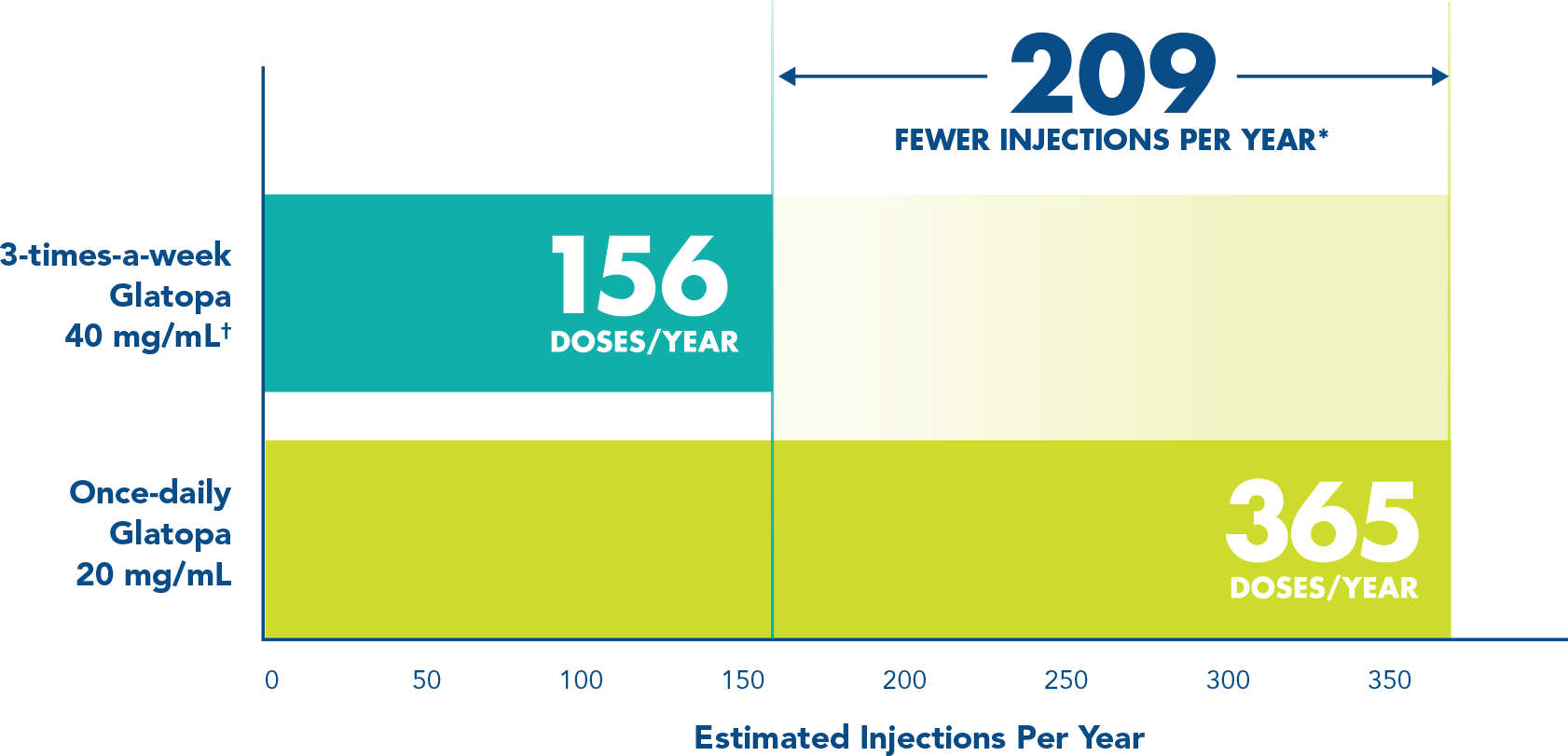
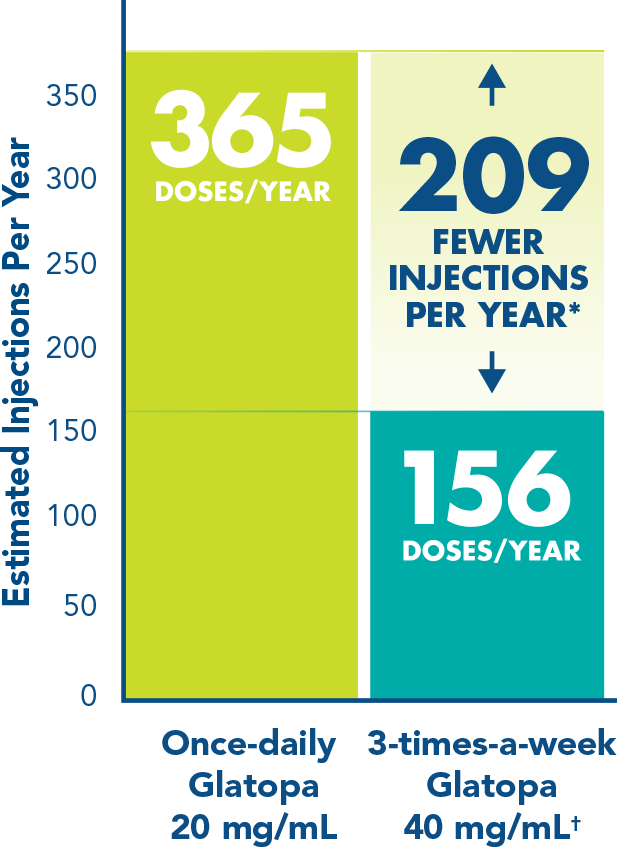
*Compared with once-daily Glatopa 20 mg/mL.
†Injections should be administered at least 48 hours apart.
Indication
Glatopa® (glatiramer acetate injection) is a prescription medicine indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
Important Safety Information
Glatiramer acetate injection is contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol.
Approximately 16% of glatiramer acetate injection 20 mg/mL patients vs 4% of those on placebo, and approximately 2% of glatiramer acetate injection 40 mg/mL patients vs none on placebo experienced a constellation of symptoms that may occur immediately (within seconds to minutes, with the majority of symptoms observed within 1 hour) after injection and included at least 2 of the following: flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, throat constriction, and urticaria. These symptoms generally have their onset several months after the initiation of treatment, although they may occur earlier, and a given patient may experience 1 or several episodes of these symptoms. Typically, the symptoms were transient and self-limited and did not require treatment; however, there have been reports of patients with similar symptoms who received emergency medical care.
Transient chest pain was noted in 13% of glatiramer acetate injection 20 mg/mL patients vs 6% of placebo patients, and approximately 2% of glatiramer acetate injection 40 mg/mL patients vs 1% on placebo. While some episodes of chest pain occurred in the context of the immediate post-injection reaction described above, many did not. The temporal relationship of this chest pain to an injection was not always known. The pain was transient, often unassociated with other symptoms, and appeared to have no clinical sequelae. Some patients experienced more than 1 such episode, and episodes usually began at least 1 month after the initiation of treatment.
At injection sites, localized lipoatrophy and, rarely, injection site skin necrosis may occur. Lipoatrophy may occur at various times after treatment onset (sometimes after several months) and is thought to be permanent. There is no known therapy for lipoatrophy.
Because glatiramer acetate injection can modify immune response, it may interfere with immune functions. For example, treatment with glatiramer acetate injection may interfere with recognition of foreign antigens in a way that would undermine the body’s tumor surveillance and its defenses against infection. There is no evidence that glatiramer acetate does this, but there has not been a systematic evaluation of this risk.
Cases of hepatic injury, some severe, including liver failure and hepatitis with jaundice, have been reported with glatiramer acetate injection. Hepatic injury has occurred from days to years after initiating treatment with glatiramer acetate injection. If signs or symptoms of liver dysfunction occur, consider discontinuation of glatiramer acetate injection.
The most common adverse reactions with glatiramer acetate injection 20 mg/mL vs placebo were injection site reactions (ISRs), such as erythema (43% vs 10%); vasodilatation (20% vs 5%); rash (19% vs 11%); dyspnea (14% vs 4%); and chest pain (13% vs 6%). The most common adverse reactions with glatiramer acetate injection 40 mg/mL vs placebo were ISRs, such as erythema (22% vs 2%).
ISRs were one of the most common adverse reactions leading to discontinuation of glatiramer acetate injection. ISRs, such as erythema, pain, pruritus, mass, edema, hypersensitivity, fibrosis, and atrophy, occurred at a higher rate with glatiramer acetate than placebo.
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information for Glatopa.
References
- Glatopa Prescribing Information. Sandoz Inc. April 2022.
- FDA approves first generic Copaxone to treat multiple sclerosis [press release]. US Food and Drug Administration; April 16, 2015.
- US Department of Health and Human Services. Orange Book: Approved drug products with therapeutic equivalence evaluations. https://www.fda.gov/media/71474/download. Accessed March 26, 2020.
- Sandoz announces US FDA approval and launch of Glatopa 40 mg/mL [press release]. Sandoz Inc. February 13, 2018.